Conservation Journal
Autumn 2014 Issue 62
A Methodology for the Conservation of Furniture Mounts
Andrew Thackray
Furniture Conservator

Figure 1. Charles Crescent Commode 1119-1882: Furniture decorated with gilt-brass mounts. Photography by Pip Barnard © Victoria and Albert Museum, London
This article outlines the approach taken by the Victoria and Albert Museum to conserve the furniture mounts of fifty objects required for display in the refurbished Europe 1600-1800 Galleries. A typical piece of furniture from this group is shown in Figure 1.
X-ray fluorescence (XRF) indicates that, in general, the alloy composition of the mounts varies, but is primarily made from an alloy of copper and zinc i.e. brass. The analysis also revealed that mounts thought to have been gilded in fact had no trace of gold.
The treatment methodology was developed following a review of the literature on historical production methods, published conservation treatments and the degradation mechanisms of the materials. Observation of corrosion patterns on similar mounts passing through the Studio also helped identify the main risks for further degradation.
Three of the main considerations behind the methodology design were as follows:
- The copper alloy substrate is vulnerable to corrosion processes. On gilt brass, this occurs through microscopic pores formed during fire gilding processes.1 Applying a coating that provides a coherent vapour barrier should reduce the exposure to airborne pollutants.
- Both gilded and non-gilded mounts may have been coloured by patination methods2 or with a tinted coating.3 The colouring of gilt mounts, termed ‘mise en couleur de l’or’, was a prized technique carried out in workshops using highly secretive and specialised methods.
- Historical natural resin coatings applied to the mounts, whether original or otherwise, are vulnerable to photochemical degradation. Voids from misapplication or unevenly applied degrading coatings can focus accelerated corrosion processes on localised areas (Figure 2).
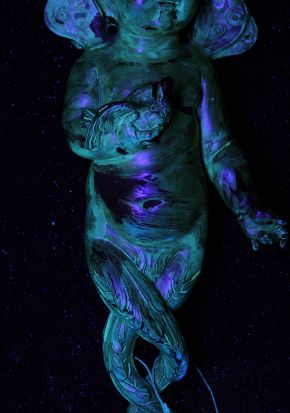
Figure 2. The surface of a mount 464-1895 viewed under UV. Photography by Andrew Thackray © Victoria and Albert Museum, London
The Museum promotes a minimal intervention approach to conservation, therefore cleaning efforts focus only on the most harmful self-catalysing salts and areas of notably disfiguring corrosion. Any patination layer, historic coating and minor areas of relatively stable corrosion are left in situ. As the parent objects have aged patinas, such as faded wood surfaces, the appearance of the treated mounts are to remain consistent with the rest of the object.
Treatment Methodology
An understanding of the materials and stratigraphy of the mount’s decorative surface is vital, as this determines the treatment choices. The mounts are therefore removed from the object for thorough inspection of all surfaces. The surfaces are viewed under UV to check for a coating and XRF analysis is carried out to identify whether or not the surface is gilded. Corrosion removal can be carried out using chemical (chelator systems) or mechanical methods, each of which has distinct advantages and disadvantages, depending on the nature of the surface being cleaned.
Localised Corrosion Removal- Chelator systems:
Chemical disassociation of specific corrosion salts may be attempted using gelled chelator systems based on bicinchoninic acid or ethylenediaminetetraacetic (EDTA) acid buffered to a pH of 9.4 This elevated pH is required for the chelator systems to efficiently sequester the most problematic copper corrosion products, however at this pH any historic natural resin coatings are likely to be compromised. Of particular concern are coatings containing pigments or dyes.

Figure 3. Cleaning tests carried out on a gilt-brass mount 1028-1882. Photography by Andrew Thackray © Victoria and Albert Museum, London
Chelator systems are seen as a less invasive means of removing corrosion, as they are more selective than mechanical methods. This ‘selectivity’ is that at a suitable pH, they will only sequester metal ions from metal salts, leaving non-corroded metal intact. In the case of cleaning furniture mounts, this selectivity may not in all cases be appropriate. It is important to remember that not all corrosion is necessarily bad. In fact, a layer of cuprite on a non-gilded mount may offer some degree of passivation to the metal layer below, and may also be valued as a patina that characterises the age of the object. On gilded mounts, any chemical colouring of the gold during manufacture or subsequent re-gilding would be removed using chelators (Figure 3). As such, tests to identify any colour change on the mount surface should be undertaken before further cleaning is carried out with chelators.
Localised Corrosion Removal- Mechanical Methods:
Spatulas can be shaped for specific contoured surfaces and used in conjunction with a chelator system or white spirit as a lubricant. The least abrasive materials are used first, moving on to harder materials if necessary (bamboo, turtle shell, ivory, mother of pearl).5 Alternatively, powdered abrasives can be applied on swabs, cotton cloth or mounted into platinum-cured silicone rubber materials. Powdered materials are used with consideration of their particle size and hardness.

Figure 4. Past abrasive treatments have worn through the gilding on this mount (object 1028-1882)
Mechanical approaches can offer more control than chemical methods, as the point of abrasion can be precisely located. They are, however, only effective when the corrosion is not as hard as the surfaces onto which they have formed. When the corrosion salts are more strongly bound to the surface, mechanical removal will inevitably risk the surfaces below, therefore a combination of chemical and mechanical methods may be more effective. In cases of particularly stubborn corrosion, a chelator system may weaken the corrosion matrix enough for mechanical methods to dislodge the structure. Mechanical methods can be used with greater control and precision than chelator systems, but if applied without caution, risk not only the removal of any coating and patination layer, but also the surface metal. On gilded mounts, this can result in loss of the thin gold layer, exposing the brass substrate below (Figure 4).
Protective Coating:
A chemically stable coating is required to provide a barrier to atmospheric pollutants. This is especially important when the surface has an historical coating. The barrier coating is applied after cleaning with hydrocarbon solvents, to remove surface dirt and accretions and the removal of any corrosion.
Paraloid™ B-72 is used for coating mounts with preserved historic natural resin coatings, as the resin is soluble in aromatic hydrocarbons. If there is no coating present, Paraloid™ B48N, a methacrylate copolymer with similar characteristics to B-72, is used, as it is specifically formulated for adhesion to metal surfaces. Acrylic coatings have been found to perform better than microcrystalline wax in tests on copper alloys exposed to simulated outdoor conditions.6 This durability and effectiveness, as a barrier to atmospheric pollutants, make for a better choice in indoor conditions too. The proprietary acrylic coating, Incralac, tested by Beale & Smith (1987), has been outperformed by B-72 after artificial aging regarding permeability to water vapour and hydrogen sulphide.7 B48N is chosen over cellulose nitrate for coating bare metals because the mounts are unlikely to be treated again in the near future; so the stability of cellulose nitrate coatings in the long term may be an issue.8
To either reduce or promote levelling of the coating, depending of the sculptural relief of the mount, the evaporation rate of the solvents used can be varied. For smaller/relief mounts, xylene for B-72 coatings or methoxypropanol for B48N coatings are used to reduce pooling in recesses. For larger/flatter mounts, slower evaporating solvents such as Shellsol A®, for B-72 or diacetone alcohol for B-48N are used to allow the applied coating to be brushed out for longer without leaving brush marks.
The common complaint against acrylic coatings making mounts appear ‘plastic’ is due to the use of unsuitable formulations of the varnish solution. Depending on whether the surface is matte or burnished, 10-20% concentrations of resin to solvent (weight by volume) produces a coherent film that can barely be apparent. Mounts are coated on all surfaces, front and back, to protect all the metal surfaces.
Thorough documentation of these treatments, along with photographs showing their current condition, will enable the long term evaluation of these treatments in the future.
Acknowledgements
I would like to thank Nigel Bamforth, Senior Furniture Conservator at the V&A and all colleagues in the Furniture Conservation Studio for their support.
References
1. Oddy, A. (1993), as cited in L. Selwyn, ‘Corrosion Chemistry of Gilded Silver and Copper’, in Drayman-Weisser, Gilded Metals, (Archetype, London, 2000) p.26.
2. Goodison, N., ‘Matthew Boulton: Ormolu’, (Christies, London, 2002) pp.142-8.
3. Tinted coatings on gilt metal mounts: Chapman, M., ‘Techniques of Mercury Gilding in the Eighteenth Century’, in Ancient & Historic Metals ed. Scott, D. Podany, J, Considine, B. (Getty Institute, Los Angeles, 1991) p.235. On non-gilded mounts: Thornton, J. ‘All that Glitters is not Gold: Other Surfaces that Appear to be Gilded’, in Drayman-Weisser, Gilded Metals, (Archetype, London, 2000) p.310.
4. Bicinchoninic acid gel: 100ml deionised water, 1g bicinchoninic acid disodium salt, 0.2g potassium sodium tartrate, add sodium bicarbonate 0.5g at a time until pH 8.5, 2g fish glue, 1ml Germaben II, 3g methylcellulose. EDTA gel: 100ml deionised water, 0.5g EDTA disodium salt, 0.5g Sodium borate, adjust pH to pH 9 with 1M sodium hydroxide, 3g methylcellulose. Recipes cited from a Richard Wolbers aqueous cleaning course, 2013.
5. Materials are sourced from museum stocks. CITIES materials can be replaced with other materials of similar hardness on the Mohs scale.
6. Beale & Smith (1987), as cited in Scott, D., Copper and Bronze in Art (Getty Publications. Los Angeles, 2002) p.358-9.
7. Selwitz, C. Cellulose Nitrate in Conservation, Getty Conservation Institute: http://www.getty.edu/conservation/publications_resources/pdf_publications/pdf/nitrate.pdf p.56.
De Witte, E 1973. ‘The protection of silverware with varnishes’, Bulletin de l’Institut royal du patrimoine artistique, 14, pp.140–151.
Autumn 2014 Issue 62
- Editorial
- Chinese export paintings
- The Clothworkers’ Foundation funding for succession training in the field of Portrait Miniatures Conservation
- Cleaning PVC with Microemulsions
- Historic surface coatings on the V&A’s plaster cast collection
- Small Stories: Dolls’ Houses Exhibition
- The Meissen Fountain: re-presenting porcelain on a grand scale
- Conservation of the Clérisseau panels
- ‘Shipping in a Calm’ by Willem van de Velde the Younger
- All that Glitters: Conservation of a Gilt Leather Chasuble
- A Methodology for the Conservation of Furniture Mounts