Conservation Journal
July 1995 Issue 16
Chemical Stabilisation of Weathered Glass Surfaces
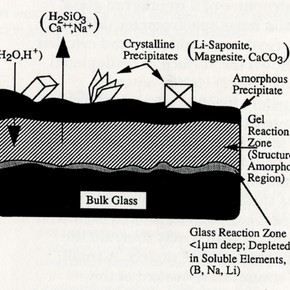
Fig. 1. Schematic diagram of the structure of a weathered glass surface. Reproduced with permission of Noyes Publications. (click image for larger version)
The conventional approach to the conservation of weathered decorative glass includes the examination, cleaning and repair and, if necessary, the consolidation and/or stabilisation of the weathered surface. These methods of conserving weathered glass have arisen largely from empirical procedures, whereby the relative efficiency and performance of various procedures and treatments have been assessed and evaluated by practising conservators. More recently, the increasingly large field of conservation science and research has challenged the intuition and experience of conservators, and has in many cases enabled a more systematic study of corrosion mechanisms, the effect of composition and environment and the long term performance of conservation treatments. This has occurred mainly through the use of a selected number of techniques including accelerated ageing and/or mathematical/computer modelling. For obvious reasons, the majority of such research is carried out on analogue samples where significant contributing factors such as raw material variability, manufacturing technology and wear from use are, for practical purposes, usually sidelined1 . However, the failure to consider both the limitations of analogue materials and the generally non-scientific experience and approach of the conservator can lead to problems. The number of factors influencing the degradation of any material is extensive, and the isolation of any one factor for study is not a trivial problem. To illustrate the point, a list of factors affecting degradation of glass objects can be seen below:
1. Raw materials - homogeneity, degree of sorting, mixing and refining;
2.Glass composition - differences between surface and bulk, differences between local or average composition, and impurities;
3.Manufacturing technology - materials preparation (grinding, mixing, etc.); processing (fritting etc.), forming and finishing, surface decoration, thermal treatment (annealing);
4.Wear from use and deterioration history - history of use and re-use, breakage, surface alteration, excavation and conservation, display and storage;
5.Environment - relative humidity, temperature (continuous or cyclic) and solution pH, composition and structure of environment, external energy factors (applied stress, electromagnetic fields, light, radiation, etc.)
The wide range of variables affecting weathering as outlined above illustrate the need for caution when interpreting results obtained from experiments. Furthermore, these factors render the use of general conservation procedures difficult, when one considers that each object has its own manufacturing, thermal and environmental history. Going to the other extreme, it is not difficult to imagine spending years of research looking at one object in extreme detail, characterising its composition and structure in terms of raw materials used, method of manufacture and corrosion characteristics, followed by an investigation of the corrosion mechanisms responsible for its decay, before the ideal conservation treatment is found and applied (not before appropriate testing and evaluation). Ideally, conservation scientists/researchers should strive to address both sides of this problem, by having a good grasp of the underlying corrosion mechanisms and formulating conservation procedures that have general applicability to objects of similar composition, age and history, without losing sight of the fact that the individual object to be treated will have unique properties and characteristics.
Such an approach has been taken in the investigation of a group of glass objects from the Museum's collection that have become crizzled. The phenomenon of crizzling has been reported in detail in a number of publications2,3,4 . Glass objects that have undergone crizzling typically have compositions that are high in alkali (soda and/or potash) and low in lime (CaO). Crizzled glass that has been investigated from the Museum's collection has a lime content of about 2-5 mol.%, compared with modern window glass that has a lime content in the order of 10 mol.%. A low silica content may also account for the instability of a glass object. The corrosion of these low lime glasses, due to chemical and physical changes in the glass surface, has resulted in the object exhibiting a range of symptoms, including loss of transparency, formation of cracks and corrosion products in the surface layers and in some cases flaking and spalling away of areas of glass from the surface.
Work is in progress, at the Materials Department at Imperial College, to investigate the corrosion mechanisms involved during the environmental weathering of analogue high soda/low lime and high potash/low lime glass compositions, with a view to first recommending conditions of safe storage and display for vulnerable and already crizzled Museum glass, and secondly looking at alternative active conservation procedures. Typical conservation procedures for such glass objects have included the use of engineered environments which aim to control the temperature and relative humidity within which the objects are stored, the regular washing of glass surfaces to remove unsightly crystalline corrosion products and the consolidation/stabilisation of flaking/spalling surfaces using a range of chemical products. However, there are a number of further potential treatments that have yet to be assessed in great detail. These include the use of laser treatment or chemical etching to reduce the surface area to volume ratio of the glass, the production of ion-stzfed surfaces utilising ion-exchange procedures which serve to produce a layer of higher density on the outermost surface, and the application of inorganic/organic - moisture barriers to the glass surface5 . Inorganic treatments may include the development of an unreactive silicate structure on the glass surface through chemical-vapour deposition or through the sol-gel method.
Another approach is the chemical stabilisation of existing weathered layers in order to reduce the reactivity of the existing surface. This approach includes the addition of inorganic or organic agents to the glass surface that reduce the solubility of the existing surface and stabilise the attacking solutions contained within pores and cracks on the surface. The application of such treatments involves the acceptance of a certain lack of reversibility in exchange for the possibility of a longer term approach to conservation. Vandiver has suggested that such an approach consists of a moral dilemma in terms of the greater risk and decreased degree of reversibility, and in making the decision about which objects should be subjected to such treatments.
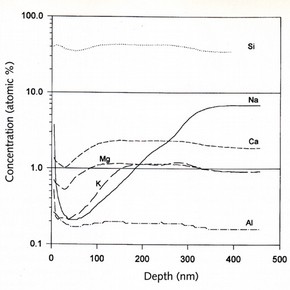
Fig.2. SIMS depth profile of a high soda/low lime glass weathered at 60% relative humidity for 72 hours at 50°C. (click image for larger version)
The current research at Imperial College is concerned with the chemical stabilisation of altered surface layers on corroded glass. Fig.l shows the structure of a weathered glass. Secondary ion mass spectrometry (SIMS) analysis of weathered analogue and crizzled glass has shown the surface to be depleted in alkali ions. (Fig.2) Unlike aqueous corrosion, where leached alkali ions are removed by the leaching solution, the weathered glass surface retains the leached alkali ions, which accumulate and may lead to salt formation. Adsorbed water molecules on the glass surface will combine with the accumulated alkali ions forming a highly alkaline solution which may then aid the dissolution of the silicate matrix. A small number of studies have looked at the chemical stabilisation of glass surface layers by altering the surface chemistry. Such studies have been approached in three ways. The first is to alter the bulk composition so as to achieve protective layers on environmental exposure, and the second is to expose the glass to hot gases during annealing to produce an almost pure silica surface. A third method is the exposure of freshly prepared materials to special solutions5 . Of these three, only the third approach has any relevance to potential conservation treatments.
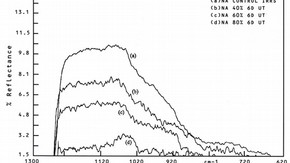
Fig.3a. Infrared reflection spectra of high soda/low lime glasses weathered for 6 days. (click image for larger version)
The presence of aluminum oxide (Al2O3) in glass slightly increases the alkaline durability6. Iler has noted that minute quantities of aluminium ions cannot only reduce the rate of dissolution of silica but, by chemisorption at the silica surface, can also reduce the solubility of silica at equilibrium7 . A number of studies have also indicated that an amorphous silica-rich phase exists on the surface of corroded glass. It is therefore reasonable to assume that a treatment whereby aluminium ions are taken up by the silica-rich surface of corroded glass will result in a more durable surface layer. SIMS, infrared reflection spectroscopy (IRKS) and X-ray photoelectron spectroscopy (XPS/ESCA) analysis of such treated analogue glass samples has revealed that aluminium ions are easily adsorbed by the weathered glass, and early trials indicate that there may indeed be some retardation of the rate of corrosion in these treated samples (Fig.3a and b).
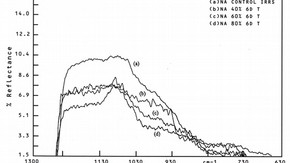
Fig.3b. Infrared reflection spectra of high soda/low lime glasses weathered for 6 days after treatment in aluminium nitrate solution. (click image for larger version)
If such approaches to conservation are successfully to become part of future conservation methods, detailed studies of the effects of these type of treatments need to be undertaken, as well as the continued research into rates and methods of corrosion of existing objects. The above represents aspects of three years postgraduate work of a V&A/Imperial College CASE studentship which I completed in June. In March, a second PhD student, Simon Hogg, started working on this project. He will aim to assess further the chemical stabilisation of glass surfaces through solution treatment, and to look at other possible long term glass conservation methods.
References:
1. Vandiver, P.B., 'Corrosion and Conservation of Ancient Glass and Ceramics'. In: 'Corrosion of Glass, Ceramics and Superconductors', Clark, D.E and Zoitos, K., eds. Noyes Publications, 1991: pp. 393-427.
2. Brill, R.H. Crizzling - 'A Problem in Glass Conservation'. In: 'Conservation in Archaeology and the Applied Arts'. IIC Proceedings, Stockholm 1975: pp. 47-68.
3. Ryan, J., McPhail, D., Rogers, P. & Oakley, V. 'Glass Deterioration in the Museum Environment'. 'Chemistry and Industry' 13, 1993: pp. 498-501.
4. Oakley, V., Rogers, P., McPhail, D & Amaku, A. 'Vessel Glass Deterioration in the Museum Environment: A Quantitative Study by Surface Analysis'. 'V&A Conservation Journal', 1992. 3: pp. 6-10.
5. Clark, D.E., Schulz, R.L., and Zoitos, B.K. In: 'Corrosion of Glass, Ceramics and Superconductors', Clark, D.E and Zoitos, K., eds. Noyes Publications, 1991: pp. 393-427.
6. Paul, A. 'Chemical Durability of Glasses: A Thermodynamic Approach'. 'J.Mats.Sci.' 12, 1977: pp. 2246-2268.
7. Iler, R.K. 'The Chemistry of Silica', John Wiley & Sons, 1979: pp. 56-58.