Conservation Journal
Summer 2005 Issue 50
Investigation of the room temperature corrosion of replica museum glass
The problem of deteriorating glass remains a serious issue for many collections around the world. It is now well known that the composition of a glass object strongly determines its properties. In many modern applications, it is common to modify the composition of the glass in order to improve its properties, for example to resist heat. However, this is also true of historical glass, where the glassmaker also manipulated the composition in order to create objects with the desired aesthetic qualities, but in doing so, unwittingly affected other properties, such as durability. The best example of this, and indeed one of the most vulnerable groups of glasses, is cristallo. Here, in the endeavour to achieve a clear crystal-like glass the raw materials were repeatedly refined.1 Analysis of these glasses has shown that the resulting compositions are high in soda and/or potash, accompanied with a very low lime content. This high alkali, low lime combination leads to a glass which readily absorbs moisture from the atmosphere.
As the objects start to absorb moisture, the glass deterioration will begin. The various stages of this corrosion process have been very well described by Oakley2 and are only summarised here. As moisture is absorbed from the atmosphere, essential elements, typically the alkalis sodium and/or potassium, are leached out of the glass and onto its surface. The leached species then form corrosion salts on the surface, leading to a dulling and loss of clarity. If the alkali is allowed to build up on the glass surface, its pH will continually increase until a point where it is so high that dissolution of the strong silica network occurs. In the most extreme case, the glass object will lose much of its mechanical strength and will eventually collapse.
In museums it is the responsibility of curators and conservators to care for these vulnerable objects and hopefully prevent their further decay. By using passive conservation, the usual approach is to display and store the objects in an environment maintained at stable temperature and relative humidity (RH) levels. A stable environment prevents the absorption/desorption cycle that occurs due to daily and seasonal changes in climatic conditions, and hopefully prevents either further moisture attack or moisture loss with consequent cracking. However, uncertainties remain as to what can be considered a 'safe' environment for these vulnerable glasses.
Recent work has focussed on the room temperature corrosion of a replica of a 16th century Façon de Venise glass composition. The composition for the replica glass was obtained from a de-accessioned piece from the V&A's collection (in weight percent: SiO2 72.72%, Na2O 17.95%, K2O 3.27%, CaO 2.17%, MgO 0.74%, Al2O3 1.21%, Fe2O3 0.23%, and Mn2O3 0.37%). The replica, copying the original object as closely as possible, was fabricated at the Royal College of Art and blown by Ian Hankey, now of Teign Valley Glass. The 'skin' formed on the glass due to the glass blowing process is an important feature of the replica material. Normally this is polished away in order to create the flat samples required for any subsequent analysis. To remove the need for polishing, large flat plates were produced.
To investigate the ageing of the replica glass under various environmental conditions, replica material was placed in chambers set to known relative humidities, also in cold storage (~8C). As a large quantity of glass was made, it was not all used in one go. It was, therefore, stored in a chamber with flowing nitrogen at room temperature. This also meant that the long term effects of storing the glass under flowing nitrogen could be examined. Previously simulated ageing has been carried out at elevated temperatures, but for these investigations the ageing was carried out at room temperature. This was made possible by the capabilities of the techniques that were subsequently employed to analyse the aged glass. This study, therefore, is one of the first that has looked at the real-time ageing of a replica museum glass, which has been fabricated using traditional methods.
The relative humidities used in the simulation experiments were 55%, 40%, 20% and 4% (dry silica gel). The samples were then left to age for known periods of time ranging from 1 day up to 45 days. After ageing, the samples were examined for the presence of corrosion salts using a three dimensional optical microscope.
It was found that after only 24 hours, corrosion salts had already formed on the surface of the glass samples aged at the highest two relative humidities. As ageing time increased, it was observed that the surface roughness and development of the corrosion salts also increased for all relative humidities, including the glass stored in the dry silica gel (4%RH). Surprisingly, to the eye, most of the samples appeared clear and would not be considered cloudy enough to require cleaning. If corrosion products are not removed from the glass surface immediately, they will continue to develop further. Work currently being carried out at Edinburgh University in conjunction with the National Museum of Scotland, has shown that the corrosion salts react with other atmospheric species such as carbon dioxide and UV, to form formate salts.3 If these surface salts are not removed they will leave behind permanent marks on an object's surface once they are removed.
As well as measuring changes occurring on the glass surface, compositional changes directly beneath the glass surface were also measured using a technique called secondary ion mass spectrometry (SIMS), this technique was also extensively used by Jason Ryan (see previous article). The sensitivity of this technique means that very small concentration changes can be measured. It is therefore possible to monitor the small changes that occur during room temperature deterioration.
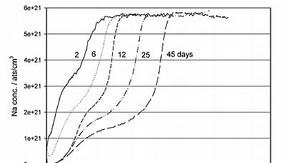
Figure 1: The development of the sodium depletion at 40%RH and room temperature for increasing ageing times from 2 to 45 days (click image for larger version)
The SIMS analysis was carried out on all the aged replica material with respect to the element sodium (Na), the alkali ion known to readily leach out of the glass during the corrosion process. Figure 1 shows an example of the changing sodium concentration for a glass aged at 40%RH and ageing times of 2, 6, 12, 25 and 45 days. The profiles show that directly below the glass surface, the sodium concentration is very much lower than the concentration of sodium deeper in the glass. This region where the sodium has been removed is known as the depletion region.
As anticipated, at increased relative humidities, the depletion depth of the sodium also increased. However, surprisingly, the depletion was also occurring at the two lowest relative humidities: 20%RH and 4%RH (the dry silica gel). These results indicate that at any given RH, sodium is leaching from the glass. Although the rate at which the sodium is leaching from the glass is slower at the lower humidities, with the slowest leaching occurring in the dry silica gel, it still occurs resulting in the formation of damaging corrosion salts on the glass surface.
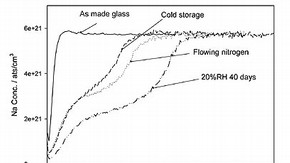
Figure 2: Sodium depletion occurring for replica glass stored in flowing nitrogen, in cold storage and a relative humidity of 20% (click image for larger version)
The effects of altering other factors in the environment were also assessed on the replica glass to see if the leaching of the sodium could be reduced or stopped. Figure 2 shows the Na depletion profiles for a glass sample that has been stored in flowing nitrogen for one year. Also shown is the Na depletion profile of another sample that has been placed in a cold storage for 40 days. Both of these profiles have been compared to the Na profile measured on the replica glass the day it was made, and a glass sample that has been aged at 20%RH for 40 days. The Na depletion profiles from the four samples are quite different. It appears that for the samples that have been placed into either a flowing nitrogen or cold environment, the leaching of the Na has been considerably slowed. This is particularly true when compared to the glass stored at 20%RH. It appears that by altering the environment to one that has either a flowing atmosphere or a lower temperature, the leaching of the sodium from the glass can be slowed down.
The funded three-year project ended in December 2004. The environmental test results came towards the end of the research and there are areas that still require clarification and further research. The development of a small test display case would allow the long term effects of specific environments to be studied, comparing, for example, the effects of an environment of flowing air with one of flowing nitrogen and considering different rates of air flow. Further tests on reducing temperatures are required to see if less dramatic temperature reduction, possibly only 5°C less than room temperature, might produce a useful reduction in the leaching of the sodium from the glass. It does appear that by altering the environment of a display case, the longevity of vulnerable glass can be improved, and there is an identified need for further research in this area.
References
1. Neri, A., 'L'Arte Vittaria', O.Pulleyn, London (1662)
2. Oakley, V., Fighting the Inevitable: the continuing search for a solution to glass decay at the V&A, 'Glass Technology' Vol. 42, No. 3 (2001) pp.65-69
3. Robinet, L., Eremin, K., Cobo del Arco, B., and Gibson, L., A Raman Spectroscopic Study of Pollution Induced Glass Deterioration, J. 'Raman Spectroscopy', 35 (2004), p.662
Summer 2005 Issue 50
- Editorial
- Rising damp - a history of the Conservation Department
- V&A Conservation on the world wide web: a secondment to the V&A web team
- The ethics checklist - ten years on
- Plastics preservation at the V&A
- Working for Diaghilev
- Pugin's wallpapers from The Grange
- Prevention is better than the cure
- Research
- The Castellani diadem
- In pursuit of a clear answer: An Exhibition Road partnership
- Investigation of the room temperature corrosion of replica museum glass
- Professional collaboration - the Prince of Wales Museum of Western India
- V&A/RIBA partnership
- Picture and mirror frames: Reflections on treatment past, present and future
- A simple solution?
- Appendix 1: Victoria & Albert Museum Conservation Department Ethics Checklist
- Conservation of a tortoiseshell book cover
- The hand that rocks the cradle: Conservation administration, present and future
- Printer friendly version