
A while back I was given six small samples to analyse – nothing unusual about this till I looked at the images of the object that the samples had come from and immediately had grand notions of treasure hunting for the Holy Grail with Indiana Jones! These thoughts were soon followed by me humming ‘knights of the round table’ down the back of the lab…

We do a lot of analysis of this type in the lab – a conservator will come to us looking to identify the materials used in a past conservation treatment. In this case the amber cup had been treated a long time ago to join the broken sections back together. Depending on the type of material used, the length of time since the treatment and the storage/environmental conditions, old conservation treatment can be in various conditions. The material used in the previous treatment of this object had become discolored and was also beginning to fail; the joints were starting to crumble away. This impacted the object in two ways – the failing joints meant the cup was structurally vulnerable and the discoloration impacted the interpretation of the object.

Now you might be wondering what the intern for modern materials is doing anywhere near an object from 17th century Prussia! When we analysed, via FTIR, the samples they mostly turned out to be amber (shocking considering the cup is made from amber and is also is used in conservation treatments) and some pigment (probably burnt sienna).

The main issue with FTIR, the scientific method we use to identify materials of this kind, is that in the resulting spectrum we see everything that was present in the sample. I spoke a little about FTIR in a previous post but didn’t delve in the world of mixtures.
If we look at the image below of two spectra from the same sample we can see that they don’t quite match up (don’t mind that the peaks have different heights this could be due to other issues like thickness of the sample used) . There are a few extra peaks in the Blue spectrum compared with the Red.
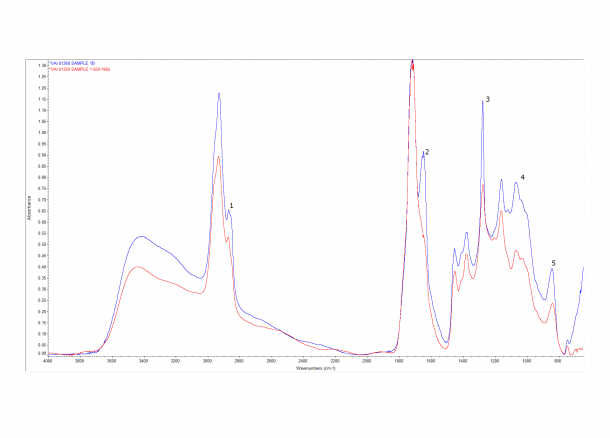
One of the easier ways we can try and distinguish mixtures is to subtract one spectrum from the other using computer software. This method isn’t perfect and a certain amount of skepticism is required – luckily this particular mixture was very straight forward. The image below shows the resulting subtraction in Red. The Purple and Green spectrum are the closest matching spectrum from our database; both are aged Cellulose Nitrate.
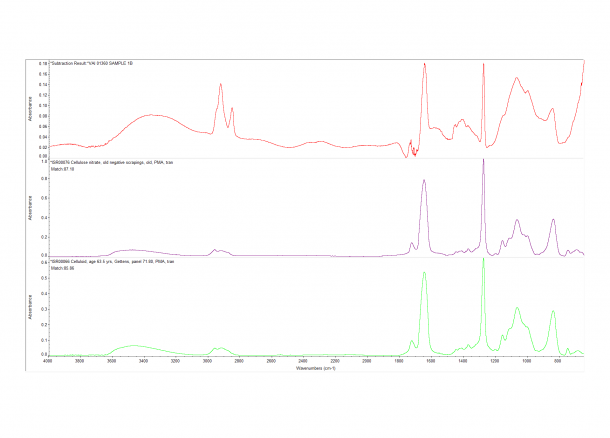
Cellulose nitrate was the first semi-synthetic “modern material” to be produced and mass marketed. In the beginning it was mostly associated with shirt collars and cuffs, and then later with film negatives; it was also used in conservation treatments and industry. However, it has a tendency to discolor, disintegrate and spontaneously catch on fire. Outside of traditional objects we commonly find cellulose nitrate used as a protective coating and adhesive in past conservation treatments. The issue conservators have is with their removal as sometimes one needs to used harsh solvents – this could have implications for the object material. The manner in which cellulose nitrate degrades can also affect the object as nitric acid is formed and this can speed up the decay of surrounding objects.


