Conservation Journal
January 1997 Issue 22
Investigations into the Use of Laponite as a Poulticing Material in Ceramics Conservation
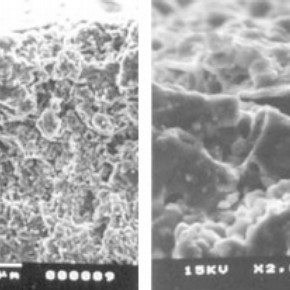
Figure 1. Surface of earthenware tile before the application of Laponire RD gel (click image for larger version)
Laponites (Laporte Absorbents) are a range of synthetic silicates manufactured from pure chemicals. Fundamentally they are closely related to the natural clay mineral hectorite [Si8Mg5.34 Li0.66 (Ca, Na)0.66], a tri-octahedral sheet silicate 1 . Industrially, Laponites are used in the manufacturing of a number of products such as paints, inks, household cleaning materials, cosmetics and shampoos. Commercially, various Laponite grades are available 2 . In recent years Laponite RD, the standard grade of Laponite, has been widely used in ceramic conservation as a simple but effective poulticing material for the removal of stains in preference to the traditional materials such as sepiolite (hydrated magnesium trisilicate), attapulgite (hydrated magnesium aluminium silicate) and paper pulp 3 . This material is available in the form of a fine white powder which readily disperses in water to produce a clear, colourless gel. The gel can be applied directly onto the surface of a stained ceramic body which has been pre-soaked in water. As the Laponite RD gel dries, soluble staining material from within the ceramic will be drawn from the object into the gel. The effectiveness of this stain leaching process is dependent on many factors which include the drying rate of the surface gel as well as its concentration, the porosity of the ceramic body and the nature of the staining substance.
Any damage due to the use of Laponite RD in stain removal techniques has not been visually evident. However, possible harmful effects caused by Laponite RD gel on the surfaces of ceramic objects are a growing concern among conservators at the V&A Museum who have reported that in comparison with non-treated objects, surfaces which have undergone the poulticing treatment are smoother to the touch and appear to be more slippery when wet. Thus, a recent research project investigating possible adverse effects of the gel on ceramic bodies has been conducted at Imperial College4 and have been continued at the Museum during the summer of 1996 5 . These studies, mainly concerned with potential damage caused by residues on treated ceramic surfaces, have confirmed the presence of residual gel on ceramics after the poulticing treatment and have shown that the amount of residual gel present depends not only on the gel concentration, the number of applications of gel onto a particular area on the object, but also on the nature of the ceramic substrate, i.e. whether the surface is glazed or porous.
Micrographs of cleaned porous earthenware tiles after the application of Laponite RD gel, shown in Figure 1, confirmed the existence of gel accumulation in and around surface pores in the ceramic substrates. This build up of residual Laponite RD resulted in a drop in the original roughness of the ceramic surface.
Fragments of lead-glazed bone china tableware and earthenware plates were treated with Laponite RD 6 , cleaned and analysed. The surface glaze on the bone china plate is much harder than that on the earthenware sample. The micrographs in Figure 2 show the surface of the ceramic samples after treatment with Laponite RD gel of concentrations 5% by weight and 9.4% by weight and reveal that the highest amount of residues were found on the earthenware ceramic substrates treated with the lower concentration gel.
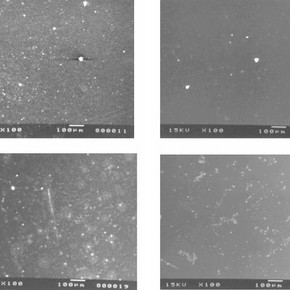
Figure 2. Surface of bone china sample after treatment with 5% by weight Laponitr RD gel (click image for larger version)
Defects present in glazes, such as scratches and abrasions, can easily become filled with debris. Thus, on applying Laponite RD, residues are likely to be present at regions near surface defects where cleaning could be more difficult. Figure 2 shows that a larger amount of residual Laponite RD is found on surfaces treated with the lower concentration, 5% by weight gel. It is believed that as the 5% by weight gel has a lower viscosity than that of the 9.4% by weight gel it can more readily flow into surface cracks and defects and would therefore be more difficult to remove.
The cleaning of deeply stained ceramics may sometimes involve several applications of Laponite RD gel onto the object before the stain is removed. Conservators at the V&A Museum speculated that repeated applications of Laponite RD gel onto the same surface may increase the amount of residual Laponite RD. Figure 3, micrographs showing the condition of glazed earthenware substrates after the application of two, three and four coats of 5% by weight Laponite RD gel confirm this hypothesis.
Figure 3. Surface of earthenware sample after treatment with two coats of 5% by weight Laponite RD gel (click image for larger version)
Secondary Ion Mass Spectroscopy (SIMS) depth profile analysis through samples of ceramic body after the removal of Laponite RD gel coatings show evidence of low level ion exchange at the interface between the Laponite RD gel coat and substrate. These depth profile plots suggest that while drawing stains from within the ceramic body, Laponite RD gel may encourage the exchange of small cations through contacting regions of gel/glaze interface. As illustrated in Figure 4, results show an unusually high level of lithium at the ceramic glaze/Laponite RD gel interface. This observation may be explained by the diffusion of lithium ions from within the gel into the surface of the glaze. As the distance, through the glaze, from the surface becomes greater, the concentration of lithium drops sharply to a depth of 0.5 m. Hence, as the thickness of the glaze on the bone china and earthenware samples are 109.5 m and 186.7 m respectively, these results reveal that the migration of small ions is restricted to within the glaze layer. As lithium migrates from the gel into the glaze, it is possible that electrical neutrality is maintained by the transportation of sodium cations in the opposite direction. This is also illustrated in Figure 4. In contrast to the high lithium content at the outer surface of the glaze, the level of sodium is unusually low and gradually increases with distance from the outer surface of the glaze.
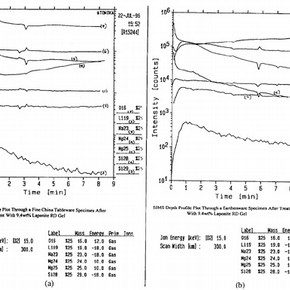
Figure 4. SIMS depth profile plot through surface of bone china sample after removal of Laponite RD gel (Relationship between time and sample depth: 1 minute = 0.04µm) (click image for larger version)
This transportation of ions between the Laponite RD gel and glaze appears to have no significant adverse effect on the ceramic body due to the low concentrations of ions involved. However, these results do confirm that the use of Laponite RD can not only alter the physical state of ceramic bodies, by the presence of residual gel, but also the chemical composition of their surface. At present, the impact of ion diffusion and the presence of gel residues on treated ceramic surfaces appear negligible. However, it should be noted that, as with many other conservation techniques, any potential long term damage may not be apparent until some time, maybe even years, after the treatment.
This short study provides an insight into the adverse effects of Laponite RD gel on surfaces of ceramic objects, in particular potential damage caused by the presence of residual gel on treated surfaces and possible diffusion of ions between a gel coating and ceramic body during treatment. The results obtained emphasise the need for continual awareness of possible long term harmful effects on ceramics when Laponite RD is used in conservation.
Acknowledgements
We are grateful to the Victoria & Albert Museum and Imperial College for providing the opportunity for this work, and to the worshipful company of Armourers and Braziers for financial support by the provision of a summer vacation bursary.
References
1.Deer, W., A., Howie, R., A., and Zussman, J., An Introduction to the Rock Forming Minerals, Longmans Group Ltd, 1983
2.Laporte Absorbents, P.O. Box 2, Moorfield Rd, Widnes, Cheshire WA1 0JU, U.K., Laponite Technical Bulletin L/RD/10/90, L/RDS/10/90, L106/93/B [PJ06], L/ED/1/94, L/DS/1/94, L/201/95j
3. Buys, S., and Oakley, V., The Conservation and Restoration of Ceramics, Butterwoth-Heineman, Oxford, 1996.
4.Lee, L-M., Laponite, its Characteristics and Its Use in Conservation Science, Final Year Project Report, Department of Materials, Imperial College, London, 1996.
5.Lee, L-M., The Use of Laponite in Ceramic Conservation, Summer Vacation Project Report, Victoria and Albert Museum, 1996.
6. Ling, D., Laponite used as a poulticing material on ceramic eathenwares at the British Museum, Conservation News 46, 1991.
January 1997 Issue 22
- Editorial
- Weighing up silver objects: evaluating past and future conservation methods
- Reflections on Silver
- Investigations into the Use of Laponite as a Poulticing Material in Ceramics Conservation
- Nappies at the National Museum of Childhood
- Mounts for the Display of Books
- The Archive of Heal & Son Limited
- Slides and Frisbees - Determining Dust Deposition Rates
- Summer Placement at the Canadian Conservation Institute
- Summer Placement at the Central Research Laboratory for Objects of Art and Science, Amsterdam
- Printer Friendly Version